Clinical Workflow Guide¶
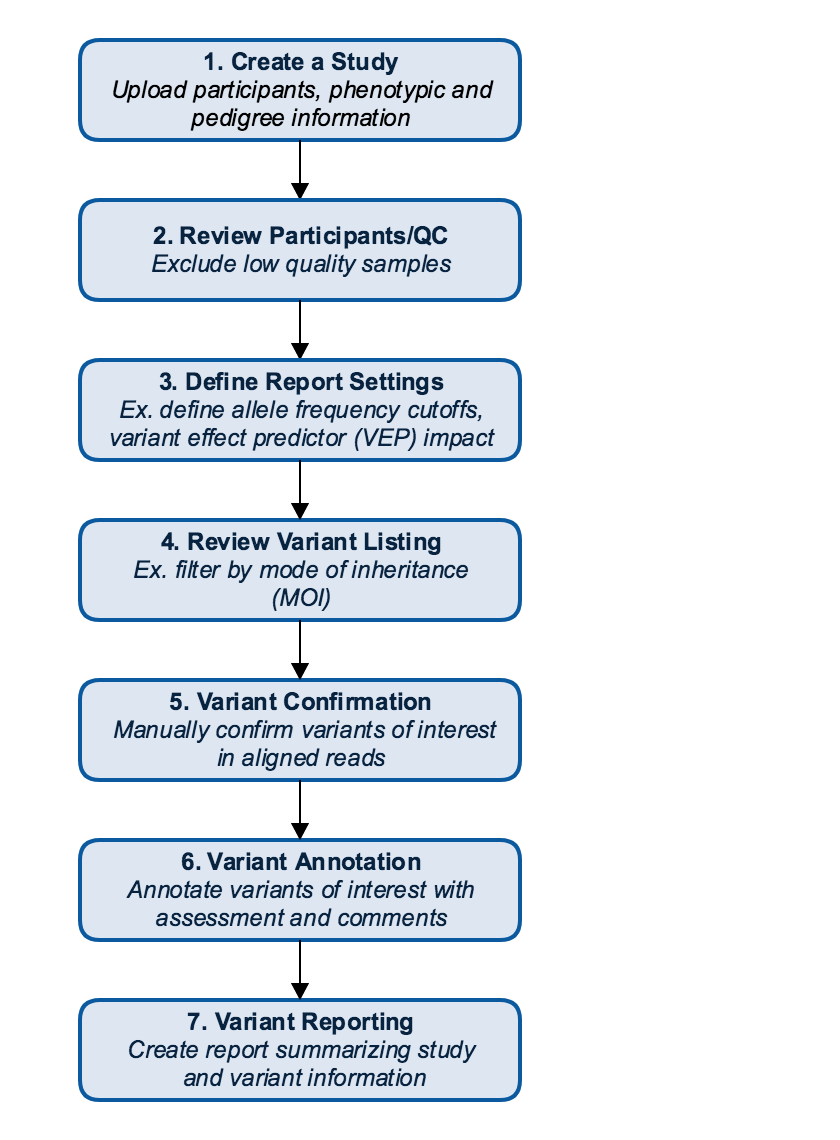
This guide walks through a typical clinical case workflow in CSA, which is usually carried out by the Variant Scientist and Lab Director user roles and includes the following steps, or workflow states:
Workflow state |
Actions |
User role |
|---|---|---|
QC Review |
Review quality metrics of the sequencing data for participants in the study |
Variant Scientist |
Case Review |
Review participants, phenotypes, and advanced report settings |
Variant Scientist |
Variant Review |
Review variant listing, add variant curations, score variants, and draft clinical report |
Variant Scientist |
Report Review |
Review and approve variant classification and clinical report |
Variant Approver and/or Lab Director |
Report Delivery |
Approve clinical report, generate PDF and deliver to physician |
Lab Director |
Case Complete |
Set the workflow state to Case Complete |
Lab Manager |
Note
Case workflows in CSA can be customized according to the lab’s needs and are configured by a CSA administrator (for more information, refer to the Administration manual).
This section covers the following topics: